Describe the Motion of Liquid Particles
As the temperature of a solid liquid or gas increases the particles move more rapidly. Conversely the motion of the particles is reduced by lowering the temperature until at the absolute zero 0 K the motion of the particles ceases altogether.
How Can You Describe The Motion Of Particles In A Solid How Can You Describe The Motion Of Particles In A Liquid How Can You Describe The Motion Of Particles Ppt
The particles have more kinetic energy.

. 120 describe the factors affecting vehicle stopping distance including speed mass road. The motion of the particles is increased by raising the temperature. What affects the direction of each particle.
Liquid-vibrate move about and slide past each other. How do particles in a gas move. Gas-vibrate and move freely at high speeds.
The motion of particles in a liquid is kinetic energy. With incresed energy liquid particles are able to. 116 know that friction is a force that opposes motion.
Describe the motion of particles in a liquid when its heated. Describe the motion of the gas particles. Does temperature not affect the movement of particles.
Describe the motion of particles in solids and the properties of solids according to the kinetic-molecular theory. Solids-vibrate jiggle but generally do not move from place to place. When a liquid gets warm the particles move faster.
Danny Landon DestinyIanTyler How do particles in liquids move. The motion of particles in a liquid is kinetic energy. In liquids particles are quite close together and move with random motion throughout the container.
Because the particles are in motion they will have kinetic energy. How do particles in a solid move. In liquids and gases the forces between the particles is weaker than forces between particles of solid.
Apply the kinetic theory of matter to explain the differences in your answer to the - 21. The particles in a liquid are close together touching but they are able to moveslideflow past each other. Particles move rapidly in all directions but collide with each other more frequently than in gases due to shorter distances between particles.
Particles in a liquid vibrate move about and. In liquids particles are quite close together and move with random motion throughout the container. 510 describe the arrangement and motion of particles in solids liquids and gases.
Describe the motion of particles in ice liquid water and water vapor. Get started for FREE Continue. The particles in gases can move freely as the forces of attraction between particles are almost negligible.
Which best describes particles in a liquid. How do particles in the plasma state move. Particles in a liquid are close together with no regular arrangement.
Basically solids have a more regular arrangement. Particles move rapidly in all directions but collide with each other more frequently than in gases due to shorter distances between particles. The particles in a liquid are close together touching but they are able to moveslideflow past each other.
Distinguish between the two types of. Note how temperature effects the motion of the atoms or molecules in a liquid. In liquid particles can slip and slide over each other.
When the particles of a liquid such as water are heated they move faster move farther apart and take up more room. The particles in a gas are fast moving and are able to spread apart from each other. As the temperature falls the particles slow down.
An in continual straight line motion the. In which state of matter are particles moving the fastest. Explanationkasalipo yung naka jiggle apsiganocj and 39.
Discuss the processes by which vaporization and freezing occur. The particles in a solid are tightly packed and locked in place. 510 describe the arrangement and motion of particles in solids liquids and gases.
The particles in a gas are fast moving and are able to. Kinetic energy of the molecules is greater than the tractive force between them therefore much further apart. Temperature directly affects the movement of particles such as molecules.
With an increasing temperature the particles gain kinetic energy and move faster the actual average speed of the particles depends on their. Describe the motion of particles in liquids and the properties of liquids according to the kinetic-molecular theory. Describe the arrangement in liquid particles.
Particles move rapidly in all directions but collide with each other more frequently than in gases due to shorter distances between particles. When the particles of a solid are heated they move faster move farther apart and take up more room. What is the motion of particles in a liquid.
In liquids particles are quite close together and move with random motion throughout the container. Describe the movement of particles in solid liquid gas and plasma states.

How Can You Describe The Motion Of Particles In A Solid How Can You Describe The Motion Of Particles In A Liquid How Can You Describe The Motion Of Particles Ppt

Motion Of Particles Flashcards Quizlet
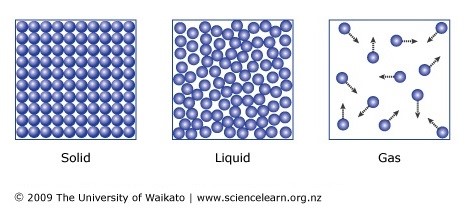
5 10 Describe The Arrangement And Motion Of Particles In Solids Liquids And Gases Tutormyself Chemistry
No comments for "Describe the Motion of Liquid Particles"
Post a Comment